info@jpsalamat.com
IPQC Islator
IPQC Islator
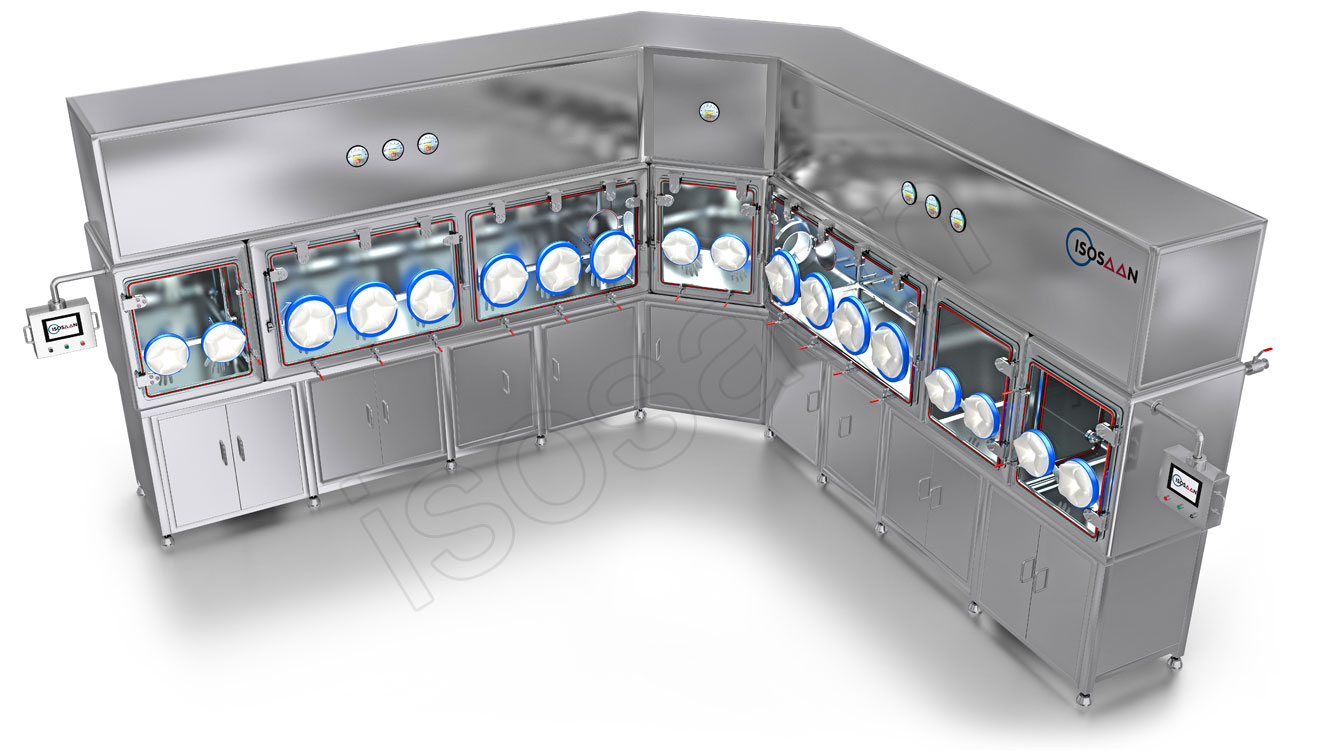
The IPQC-Isolator device is an advanced containment system used in In-Process Quality Control (IPQC) to ensure a sterile and contamination-free environment during pharmaceutical manufacturing. This device provides a highly controlled atmosphere by using HEPA or ULPA filtration, maintaining positive or negative pressure, and ensuring a physical barrier between the product and external contaminants.
IPQC-Isolators are widely used in the production and testing of sterile drugs, biologics, and hazardous compounds, where strict contamination control is required. They allow operators to handle materials through glove ports, reducing direct human contact and minimizing the risk of microbial or particulate contamination. These isolators comply with Good Manufacturing Practice (GMP) and ISO 14644 standards, making them essential in sterile drug production, quality assurance, and microbiological testing in pharmaceutical and biotech industries.
IPQC-Isolators are widely used in the production and testing of sterile drugs, biologics, and hazardous compounds, where strict contamination control is required. They allow operators to handle materials through glove ports, reducing direct human contact and minimizing the risk of microbial or particulate contamination. These isolators comply with Good Manufacturing Practice (GMP) and ISO 14644 standards, making them essential in sterile drug production, quality assurance, and microbiological testing in pharmaceutical and biotech industries.